Bringing new drugs to the market is a time-intensive and insanely expensive process exposed to high risk of failure. And Digital Health startups play a major role to facilitate testing new drugs during clinical trials.
In a recent report, the World Health Organization (WHO) informs that chronic diseases, such as cardiovascular diseases, cancers, diabetes and neurological disorders cause about 15 million deaths each year for people aged 30 to 70. In the last 50 years, pharmaceutical research has produced more drugs than ever before. And this progress continues with the development of drugs that are still better adapted to the genetic profile of patients. This drug development is a long and expensive process that faces various challenges. I mapped out below the different stages in pharma drug production.
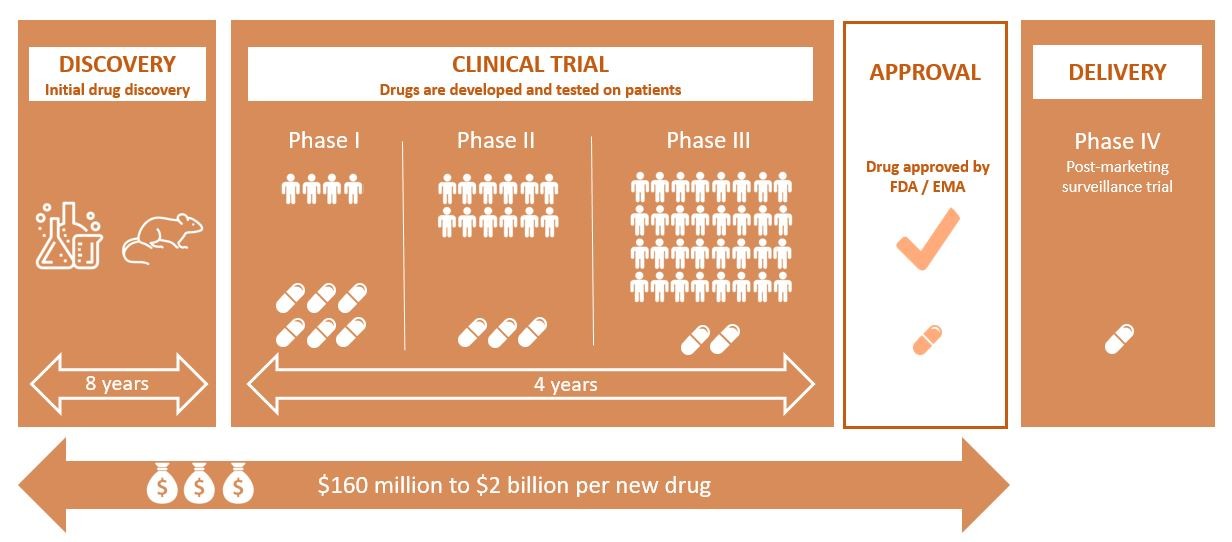
Before being commercialized, a new drug has to pass various tests. After being discovered and tested at the lab, the drug is being tested on humans: it is the clinical trials. In a report led by Grand View Research, the global clinical trials market is expected to reach $65.2 billion by 2025. Clinicaltrials.com lists more than 290,000 active clinical trials studies in 207 countries.
Only 1 in 10 drugs that enter clinical trials make it to market.
Drugs usually fail when considered to be too dangerous or not enough efficient. But half of the drugs rejected fail for other reasons, such as failure to recruit enough participants, mid-trail patient drop out, and poor data collection methods. Hundreds of startups try to disrupt and modernize this market. Here are 4 ways how startups help clinical trials being more efficient, faster or less expensive.
Digital Health for monitoring patient data collection
Converting hospital paper files to Electronic Health Records (EHRs) has been widely adopted all over the world by healthcare providers. It facilitates data collection and enables to share patient information much faster than before. Even though EHRs still face major challenges including the lack of interoperability, high cost and privacy considerations, it makes it possible for AI startups to analyze medical records and suggest eligible patients within minutes - a process that would otherwise take months. For example, MDClone has developed a technology that preserves privacy of patient health data while allowing researchers to use it. On the one hand, the company collects patient information from hospitals using its software. On the other hand, MDClone enables biopharma companies to identify patients for clinical trial enrollment. MDClone is already well established in the USA and is now extending to Europe.
AI for matching eligible patients to the right clinical trials
When it comes to non-conventional treatments and clinical trials, it is nearly impossible for most patients to navigate and truly understand their options. Biopharma companies also find it difficult to find eligible patients. The matching process is very demanding in terms of eligibility criteria. Exclusion and inclusion criteria are often explained in medical jargon and include historical data, previous surgeries, lab test, medical imaging results, drug interactions, allergic reactions, genetic information.
According to Camille Vever, Keyrus Biopharma CEO, nearly 80% of clinical trials fail to meet patients’ enrollment. TrialJectory is developing a solution based on machine learning and AI to enroll cancer patients with the right clinical trials. Instead of a list of a few hundred clinical trials, the patient will end up with two, three, or five clinical trials that are relevant to him. Once the patient selects one, the company contacts the pharmaceutical company running the chosen trial to arrange for participation. The platform already placed about many patients to the right trials.
Medication adherence solutions to greater patient retention
Once a patient enrolls in a clinical trial, he receives specific instructions on the studied drug, the dosage and the time to take it. Especially in the field of chronicle diseases, multiple and sophisticated medications can lead to adverse effects on the patient health, accidental overdose or ineffective treatment. In addition, it decreases the accuracy of study outcomes and can incur huge costs for the medical study. Biopharma companies rely on patient memory and engagement, which are hard to control/measure.
Medisafe’s mobile app help patients better manage medication doses. The solution includes reminders, motivation systems, targeted content, coupons and interventions in the local language. The company is backed by Merck which participated in Medisafe’ Series B funding. This partnership will help improve medication adherence for Merck’s cardiometabolic patients in Brazil, Russia and Mexico. Another startup in this category, Vaica, developed smart dispensers that can be customized by pharmaceutical companies to ensure the medication was taken at the right time or the right dose was administrated. From inhalers to injectables, the device was designed to encourage patients with reminders.
Remote monitoring to lower patient and trials site burden
Frequent travel to a trial site, regular check-ins and daily report are also very time consuming and can reduce patient retention. Some patients just give up. Remote monitoring is a method aimed to gather and monitor data from a patient (in this case) without being in contact with him. I explored the topic of remote sensing in its broadest definition in a previous article.
Medical wearable that collect patient data remotely are already a core part of Sanofi and Pfizer’s digital trials strategies.
ContinUse Biometrics developed a smart sensor measuring continuously and remotely 20+ biomedical parameters used during clinical trials. The solution, based on nanotechnology and AI, detects molecular vibration with no physical contact. The company raised a $27 million to date and is partnering with leaders in healthcare. The data collected is shared automatically with the healthcare team, and reduces the number of visits and reports. ContinUse Biometrics enhances the well-being of subjects and increase patient retention.
Another startup, Datos Health, developed an AI-based platform for remote data management on a large scale. The solution collects data from any medical device, including heart rate, temperature, sleep, glucose level and blood pressure. Clinicians can send notifications and demands to the patients in case of abnormal activity or to adjust medications. Datos has proven significant efficiency in diabetes type 2 and lung cancer. I observed that a large amount of solutions initially designed for hospitals and chronic patients find high value use cases working with biopharma companies. In my opinion, digital technologies that are leveraging patient data, are going to be part of the future of clinical trials, resulting in a decrease in the cost of trials.